Lessons from nature
Energy economy of living organisms
What is the fuel of living organisms?
Sunlight is the primary energy source in our world and photosynthetic organisms use it to perform chemical reactions in a controlled fashion with a very complex machinery that is yet to be fully explained. Stimulated by light harvesting, a cascade of physical and chemical events are responsible to perform the “splitting of water” into dioxygen (O2) and hydrogen atoms (storage as NADH inside cells) finally storing solar energy in chemical bonds. Since the products are thermodynamically less stable than water, in a different biological cycle, dioxygen is reused as an electron acceptor, yielding water as a by-product and closing the energy economy of the cell.
The molecular machines of living organisms
Photosystem II
The oxidation of two water molecules is coupled to an electron flow induced by absorption of photons in chloroplasts. The overall reaction is:
2H2O → 4H+ + 4e- + O2
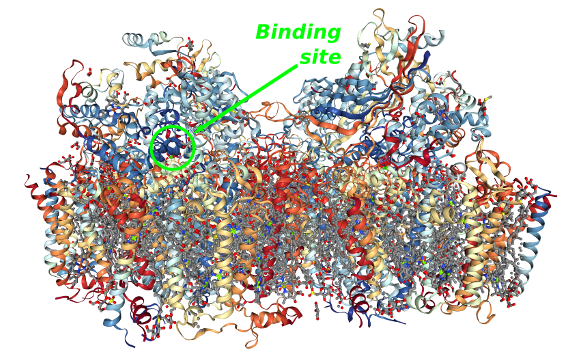
All this process takes place in the Photosystem II, a dimeric membrane protein complex composed by 20 subunits (figure above) described in detail by Umena et al in 2011 .
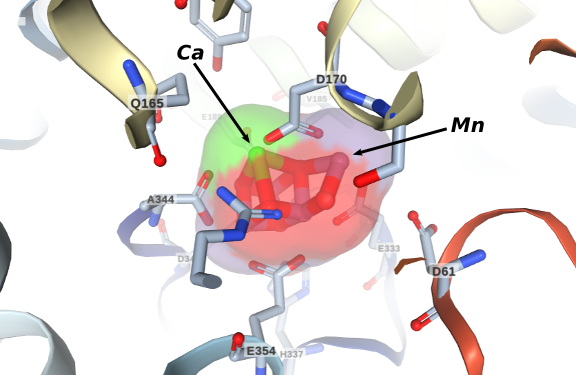 Four electrons are extracted from two water molecules in the active site known as the oxygen-evolving complex (OEC) shown in the figure in the left. It is composed by a manganese-calcium cluster normally represented by the formula Mn4CaO5. The understanding of its operation is an ongoing problem. It is believed that its most important structural characteristic is its distorted chair configuration, a consequence of the presence of the calcium ion. The mechanism of water activation by the OEC is yet unknown and several important groups are dedicated to unveil this very important question .
Four electrons are extracted from two water molecules in the active site known as the oxygen-evolving complex (OEC) shown in the figure in the left. It is composed by a manganese-calcium cluster normally represented by the formula Mn4CaO5. The understanding of its operation is an ongoing problem. It is believed that its most important structural characteristic is its distorted chair configuration, a consequence of the presence of the calcium ion. The mechanism of water activation by the OEC is yet unknown and several important groups are dedicated to unveil this very important question .
Cytochrome C Oxidase
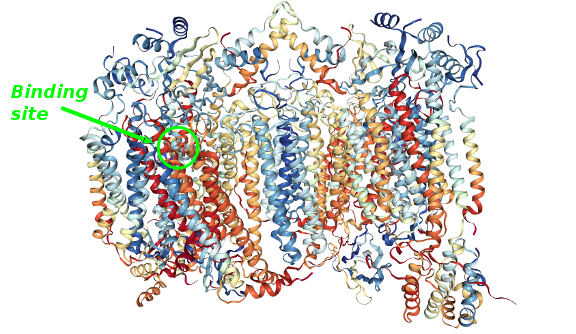
The final stage of the respiratory chain inside cells takes place at an enzymatic complex known as the Cytocrome-c Oxidase or Complex IV. A cascade of redox reactions ends in this complex in which four electrons are transfered to dioxygen generating water. The structure and function of this important enzymatic complex was discovered by many scientists over the years specially by biochemists, inorganic chemists and crystallographers.
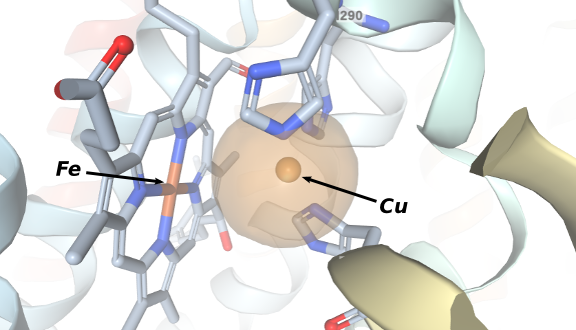 The crystal structure of the 13-subunit oxidized cytochrome c oxidase was described in 1996 by Tsukihara et al . This is the resting state of the enzyme and it revealed in great detail the reaction site where dioxygen coordinates. The figure in the right shows that an iron(III) protoporphyrinate is positioned in front of a Cu(II) ion coordinated to three residues of histidine. Other structures were obtained and several models of the enzyme were synthesized in the mid-1990s specialy by the group of Prof. Collman from Stanford University .
The crystal structure of the 13-subunit oxidized cytochrome c oxidase was described in 1996 by Tsukihara et al . This is the resting state of the enzyme and it revealed in great detail the reaction site where dioxygen coordinates. The figure in the right shows that an iron(III) protoporphyrinate is positioned in front of a Cu(II) ion coordinated to three residues of histidine. Other structures were obtained and several models of the enzyme were synthesized in the mid-1990s specialy by the group of Prof. Collman from Stanford University .
All these collected results suggest that the resting state must be reduced by two electrons to allow dioxygen coordination and when this happens a peroxo-bridge is formed between Fe(III) and Cu(II). The following steps are proton-coupled electron transfers, transforming the peroxo bridge into two water molecules, regenerating the resting state. The overall reaction is the proton-coupled tetraelectronic reduction of dioxygen:
4H+ + 4e- + O2 → 2H2O